PSMA-TRACTr (JANX007)
In May 2025, Janux announced initiation of its Phase 1b expansion studies in taxane-naïve mCRPC patients.
JANX007 is Janux’s lead novel Tumor Activated T Cell Engager (TRACTr). JANX007 is designed to target PSMA, a protein expressed in prostate cancer tumors and the vasculature of tumors and is in the clinic for the treatment of metastatic castration-resistant prostate cancer (mCRPC).
Prostate cancer is the second most common cancer in men, leading to over 34,000 deaths in the United States annually. PSMA is known to be highly expressed in prostate cancer which has led to the development of PSMA-targeted biologics, including T cell engagers (TCEs). A third-party clinical trial with a continuously infused PSMA-TCE (no longer in development) demonstrated clinical benefit, suggesting the potential of a PSMA-TCE approach.
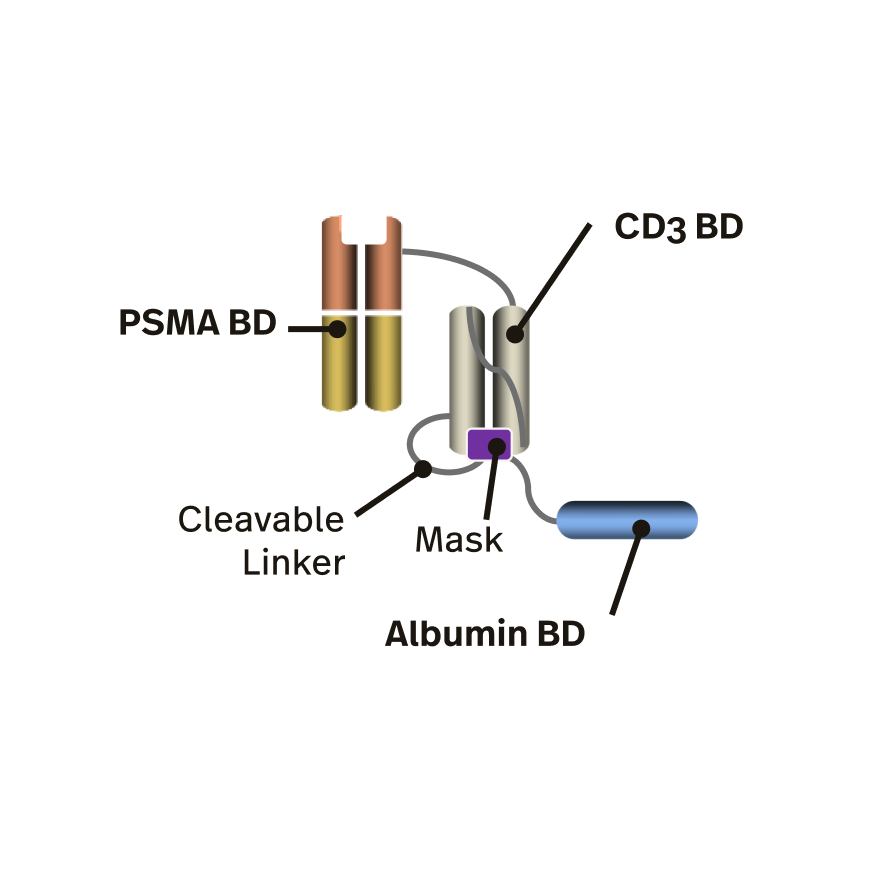
Unlike existing traditional TCE approaches to prostate cancer that have been limited to-date by dose-limiting toxicities, poor pharmacokinetic (PK) profiles and attenuated efficacy, JANX007 is designed as a highly potent and safer anti-tumor approach to mCRPC.